A Comprehensive Guide (2024 Trends Included)
In the interesting world of chemistry, understanding how atoms interact, bond, or repel each other is crucial for grasping the underlying principles that dictate chemical reactions. One key concept that helps explain these behaviors is ionization energy. Ionization energy refers to the amount of energy required to remove an electron from an atom or ion in its gaseous state. This property plays a fundamental role in determining the reactivity of elements, especially when forming ionic or covalent bonds. It is also pivotal in various scientific fields such as material science, quantum chemistry, and even astrophysics.
Ionization energy provides insight into an atom’s stability, as atoms tend to resist losing electrons. For instance, noble gases, like neon and argon, have high ionization energies, making them stable and inert. On the other hand, alkali metals, like sodium and potassium, have low ionization energies, which explains their high reactivity. By examining ionization energy trends, scientists can predict how elements will behave in a chemical reaction or how they may combine with other atoms.
In 2024, the study of ionization energy has evolved, thanks to advanced computational methods and novel experimental techniques. Researchers are increasingly able to fine-tune predictions about ionization energies and use this knowledge to develop new materials, technologies, and sustainable energy solutions. Understanding ionization energy is no longer just theoretical but has practical implications in fields ranging from nanotechnology to clean energy.
Table of Contents
A Comprehensive Guide (2024 Trends Included)
Factors Affecting Ionization Energy.
Trends in Ionization Energy Across the Periodic Table.
The Role of Ionization Energy in Chemical Bonding.
Ionization Energy and Its Role in Periodic Trends.
Trends in Ionization Energy in 2024: Emerging Research and Implications.
Top 20 Frequently Asked Questions (FAQs) on Ionization Energy.
This article will delve into the concept of ionization energy, its factors, trends in the periodic table, and how the latest advancements in 2024 have impacted this field. Whether you’re a student, researcher, or chemistry enthusiast, this guide aims to provide a clear and in-depth exploration of ionization energy, its significance, and its real-world applications.
What is Ionization Energy?
Ionization energy is the minimum energy required to remove an electron from a neutral atom or ion in its gaseous state, resulting in a positively charged ion. The process of ionization can be represented as:
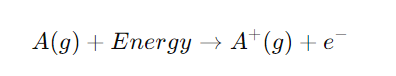
A(g)+Energy→A+(g)+e−A (g) + Energy → A^+ (g) + e^-A(g)+Energy→A+(g)+e−
Here, AAA represents the atom, and A+A^+A+ is the resulting ion after the electron e−e^-e− is removed. The unit of ionization energy is typically expressed in electron volts (eV) or kilojoules per mole (kJ/mol). Ionization energy is crucial because it provides insights into an atom’s electron configuration, reactivity, and bonding behavior.
There are different types of ionization energies depending on how many electrons are removed:
- First Ionization Energy (IE1): The energy required to remove the first electron.
- Second Ionization Energy (IE2): The energy required to remove a second electron from the resulting ion.
- Third Ionization Energy (IE3), and so on.
As each successive electron is removed, the ionization energy increases. This happens because once an electron is removed, the remaining electrons experience a greater effective nuclear charge (attractive force from the nucleus), making it harder to remove the next one.
Factors Affecting Ionization Energy
Several factors influence ionization energy, and understanding these can help explain the variations in this property across different elements.
- Nuclear Charge: The greater the nuclear charge (number of protons), the stronger the attraction between the nucleus and the electrons. This makes it more difficult to remove an electron, thus increasing the ionization energy. For example, helium has a higher ionization energy than hydrogen because it has more protons.
- Atomic Radius: As the atomic radius increases, the distance between the nucleus and the outermost electrons increases as well. This weakens the attraction between the nucleus and electrons, making it easier to remove an electron, thus lowering the ionization energy. This explains why ionization energy decreases as you move down a group in the periodic table.
- Electron Shielding: Inner electron shells act as a shield, reducing the effective nuclear charge experienced by the outermost electrons. The more electron shells an atom has, the lower the ionization energy because the outer electrons are less tightly bound to the nucleus.
- Electron Configuration: Atoms with stable electron configurations, such as noble gases, have high ionization energies because removing an electron would disturb their stability. In contrast, alkali metals, which have one loosely bound electron in their outermost shell, have low ionization energies.
- Penetration Effect: Electrons in orbitals closer to the nucleus (such as s-orbitals) experience a greater attraction to the nucleus than those in orbitals farther away (such as p- or d-orbitals). As a result, electrons in s-orbitals are harder to remove, increasing the ionization energy.
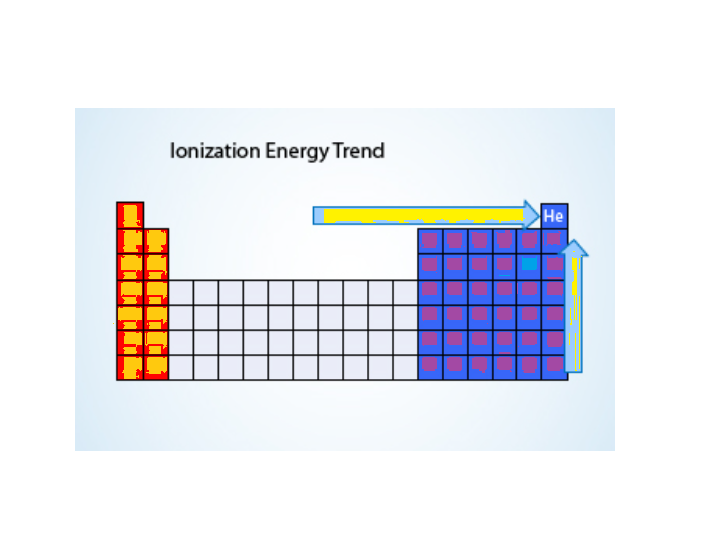
Trends in Ionization Energy Across the Periodic Table
Ionization energy exhibits clear trends across the periodic table, both within periods (rows) and groups (columns).

Python: The Complete Reference Paperback – 20 March 2018
by Martin C. Brown (Author)
1. Ionization Energy Across a Period (Left to Right):
- As you move from left to right across a period, the ionization energy increases. This is because the nuclear charge increases while the atomic radius decreases, pulling the electrons closer to the nucleus and making it harder to remove them.
- For example, in Period 2, lithium (Li) has a lower ionization energy compared to neon (Ne), which has a much higher ionization energy. This is because neon has a full outer shell of electrons, making it stable and resistant to losing electrons.
2. Ionization Energy Down a Group (Top to Bottom):
- As you move down a group, the ionization energy decreases. This is due to the increase in atomic radius and electron shielding. The outermost electrons are farther from the nucleus and are more shielded by inner electrons, reducing the attraction between the nucleus and the outermost electrons, making them easier to remove.
- For instance, in Group 1 (the alkali metals), lithium (Li) has a higher ionization energy compared to cesium (Cs), which has a much lower ionization energy because its outermost electron is farther from the nucleus.
3. Exceptions in Ionization Energy Trends:
- Although general trends exist, there are exceptions. For example, elements in Group 13 (boron family) have slightly lower ionization energies than expected due to the presence of p-orbitals, which are easier to remove electrons from than s-orbitals.
- Similarly, oxygen has a slightly lower first ionization energy than nitrogen, even though it is farther to the right in the periodic table. This is because of the electron-electron repulsion in oxygen’s p-orbitals, which makes it easier to remove an electron.

Successive Ionization Energies
Ionization energy increases significantly with the removal of each successive electron. This is because, with each electron removed, the remaining electrons are more tightly bound to the nucleus due to a stronger effective nuclear charge. For instance, the second ionization energy of sodium is much higher than its first because removing an electron from the 1s^2 2s^2 2p^6 (a stable, noble-gas-like configuration) is significantly harder than from its initial 3s^1 state.
The Role of Ionization Energy in Chemical Bonding
Ionization energy plays a pivotal role in determining how atoms interact and bond with one another. Elements with low ionization energies tend to lose electrons easily, making them more likely to form cations (positive ions), as seen in metals. Conversely, elements with high ionization energies, such as non-metals, are less likely to lose electrons and are more inclined to gain electrons, forming anions (negative ions).
This concept helps explain the formation of ionic bonds. In an ionic bond, a metal atom with low ionization energy donates one or more electrons to a non-metal atom with high electron affinity, creating a stable compound. For example, sodium (with a low ionization energy) easily loses an electron to form Na^+, while chlorine (with a high electron affinity) gains that electron to form Cl^-, resulting in the formation of NaCl (table salt).
Ionization Energy and Its Role in Periodic Trends
Ionization energy is a critical factor in understanding periodic trends. Along with electron affinity, atomic radius, and electronegativity, it helps scientists predict how atoms will behave in chemical reactions and how they will bond with other atoms.
For instance, in predicting reactivity, elements with low ionization energies (such as alkali metals) are typically highly reactive because they can lose their outermost electron easily. Conversely, noble gases, with their high ionization energies, are chemically inert under normal conditions.
Additionally, ionization energy is important in explaining the color of transition metal complexes, the formation of ions in mass spectrometry, and even the chemical properties of elements in various states.
Trends in Ionization Energy in 2024: Emerging Research and Implications
As we move into 2024, advances in technology and computational chemistry have opened new doors for understanding ionization energy. One major development is the use of machine learning models to predict ionization energies with unprecedented accuracy. These models are trained on large datasets of elements and compounds, enabling scientists to predict ionization energies for new, untested materials. This has significant implications for fields such as materials science, where predicting the ionization energies of novel alloys or compounds can lead to the development of better batteries, catalysts, and semiconductors.
Moreover, researchers are delving deeper into the ionization energies of elements under extreme conditions, such as high pressure or temperature, to understand how their chemical properties might change. These studies are particularly important for planetary science and astrophysics, where elements in distant exoplanets or stars might behave differently than they do on Earth.
Finally, with the increasing focus on sustainability and green chemistry, understanding ionization energy is crucial in developing energy-efficient processes. For instance, finding materials with low ionization energies that can efficiently donate electrons could lead to breakthroughs in photovoltaic cells or fuel cells, contributing to cleaner energy production.
Top 20 Frequently Asked Questions (FAQs) on Ionization Energy
Here is a list of top 20 most common FAQs on ionization energy. These FAQs provide a comprehensive understanding of ionization energy, covering fundamental concepts, trends, and its relevance in modern science and technology.
- What is ionization energy?
- Ionization energy is the minimum amount of energy required to remove an electron from an atom or ion in its gaseous state. It reflects how tightly an electron is held by an atom’s nucleus and is expressed in electron volts (eV) or kilojoules per mole (kJ/mol).
- Why is ionization energy important?
- Ionization energy helps us understand an atom’s reactivity, chemical bonding behavior, and stability. It indicates how easily an atom can lose or gain electrons, which is critical for predicting chemical reactions, forming compounds, and determining material properties.
- What is the difference between the first, second, and third ionization energies?
- The first ionization energy is the energy required to remove the first electron from a neutral atom. The second ionization energy refers to the energy needed to remove a second electron from a positively charged ion, and the third ionization energy applies to the removal of the third electron. Each subsequent ionization energy is higher because the remaining electrons are more strongly attracted to the nucleus.
- How does atomic size affect ionization energy?
- Atomic size is inversely related to ionization energy. As atomic size increases, the outermost electrons are farther from the nucleus, experiencing weaker attraction, making it easier to remove an electron and lowering the ionization energy.
- What are the main factors that influence ionization energy?
- Several factors influence ionization energy:
- Nuclear charge (more protons increase ionization energy),
- Atomic radius (larger atoms have lower ionization energy),
- Electron shielding (inner electrons shield outer electrons, reducing ionization energy), and
- Electron configuration (stable electron arrangements have higher ionization energies).
- Several factors influence ionization energy:
- Why does ionization energy increase across a period in the periodic table?
- Ionization energy increases across a period from left to right due to increasing nuclear charge (more protons), which pulls electrons closer to the nucleus, making them harder to remove. The atomic radius decreases, but the electron shielding effect remains relatively constant across a period.
- Why does ionization energy decrease down a group in the periodic table?
- As you move down a group, ionization energy decreases because atoms have more electron shells, increasing the distance between the nucleus and the outermost electrons. The increased electron shielding from inner electrons also reduces the effective nuclear charge, making it easier to remove an electron.
- What are some exceptions to the periodic trends in ionization energy?
- While ionization energy generally increases across a period and decreases down a group, there are exceptions. For instance:
- Group 13 elements have slightly lower ionization energies than expected due to the p-orbital electron being easier to remove.
- Oxygen has a lower first ionization energy than nitrogen because of increased electron-electron repulsion in oxygen’s half-filled p-orbitals.
- Why does the second ionization energy of an element tend to be higher than the first?
- The second ionization energy is higher because after the first electron is removed, the atom becomes a positively charged ion. The remaining electrons experience a stronger attraction to the nucleus, making it harder to remove a second electron.
- What is the relationship between ionization energy and reactivity?
- Elements with low ionization energies (e.g., alkali metals) are more reactive because they lose electrons easily, forming positive ions. Conversely, elements with high ionization energies (e.g., noble gases) are less reactive because it requires much more energy to remove an electron.
- How does electron shielding affect ionization energy?
- Electron shielding occurs when inner electrons block the outermost electrons from the full attraction of the nucleus. More electron shielding reduces the effective nuclear charge felt by the outer electrons, lowering the ionization energy and making it easier to remove an electron.
- What is the trend in ionization energy among alkali metals?
- Alkali metals (Group 1 elements) have low ionization energies, which decrease as you move down the group. This is because the atomic radius increases, and electron shielding becomes more significant, making it easier for alkali metals to lose their outermost electron.
- Why do noble gases have the highest ionization energies?
- Noble gases have the highest ionization energies because they have a full valence shell of electrons, making them stable. Their atoms strongly resist losing electrons, and their small atomic radius and lack of electron shielding result in very high ionization energies.
- What is the difference between ionization energy and electron affinity?
- Ionization energy measures the energy required to remove an electron from an atom, while electron affinity measures the energy change when an atom gains an electron. Ionization energy reflects how strongly an atom holds onto its electrons, whereas electron affinity indicates how much an atom “wants” to gain an electron.
- Can ionization energy be negative?
- No, ionization energy is always positive because energy must be supplied to remove an electron from an atom. A negative value would imply that energy is released when removing an electron, which contradicts the definition of ionization energy.
- How does ionization energy relate to ionic bonding?
- In ionic bonding, elements with low ionization energies (usually metals) lose electrons and form positive ions (cations), while elements with high electron affinity (usually non-metals) gain electrons to form negative ions (anions). The difference in ionization energy and electron affinity between two atoms helps predict the formation of ionic bonds.
- How is ionization energy measured?
- Ionization energy is typically measured through spectroscopy, particularly photoelectron spectroscopy, where photons are used to eject electrons from an atom. The energy required to remove the electron is then measured, allowing for the calculation of ionization energy.

- What is the significance of ionization energy in spectroscopy?
- Ionization energy is crucial in spectroscopy because it helps identify elements based on the energy required to remove electrons. In photoelectron spectroscopy, for instance, ionization energies are used to determine the electronic structure of atoms and molecules.
- What role does ionization energy play in astrophysics?
- In astrophysics, ionization energy helps explain the behavior of atoms under extreme conditions, such as in stars or interstellar space. Understanding ionization energies is essential for studying the ionization states of elements in stellar atmospheres, which affects star temperature, radiation, and chemical composition.
- How are ionization energies used in modern research and technology?
- In 2024, ionization energy continues to be crucial in research areas such as materials science, nanotechnology, and clean energy. Predicting ionization energies of new compounds helps in designing better catalysts, batteries, semiconductors, and solar cells. Advances in machine learning and computational chemistry have improved our ability to predict ionization energies, speeding up the discovery of new materials.
Conclusion
Ionization energy is a fundamental concept in chemistry that provides essential insights into the behaviour, reactivity, and bonding of atoms. By understanding the factors that influence ionization energy and the trends across the periodic table, chemists can make informed predictions about chemical reactions, bonding patterns, and material properties.
As we move forward into 2024, the study of ionization energy continues to evolve, with advances in computational techniques, machine learning, and experimental technologies opening up new possibilities for research and application. From developing new materials to contributing to clean energy solutions, the understanding of ionization energy remains a cornerstone of both theoretical and practical chemistry.
Curated Reads:




![[keyword]](https://learnxyz.in/wp-content/uploads/2026/02/Accelerate-open-source-development-with-AI.jpg)
Awesome! Its genuinely remarkable post, I have got much clear idea regarding from this post .
Family Dollar This is my first time pay a quick visit at here and i am really happy to read everthing at one place